We were so excited to welcome many of our members in person to Orlando for our Midyear Conference, but once again, we also offered the opportunity to attend virtually through our hybrid conference format. We were delighted to have so many attendees join us both in Florida and from home!
Remember, if you missed any of the exciting and informative sessions, or if you just want to revisit them, all of the meeting content (including the live “chats”) is archived and accessible to AONN+ members for an entire year. So whether you’re a nurse or patient navigator, a social worker or financial navigator, an administrator, or you just want to learn more, there are opportunities for everyone to further their education!
- Publishing Your Navigation Research: From Abstract to Print
- “What’s in It for Me?” Helping Patients Understand Oncology Clinical Trials
- Defining New Roles: What’s an Oncology Clinical Trial Nurse Navigator?
Publishing Your Navigation Research: From Abstract to Print

Publishing your navigation research may seem like a daunting task, but becoming familiar with the step-by-step process involved in getting published can make it a more manageable and realistic undertaking, according to Kristin Siyahian, Editorial Director of the Journal of Oncology Navigation and Survivorship (JONS).
AONN+ has a very specific goal: to elevate the cause of navigation, to elevate the role of navigators on care teams, and to highlight navigation tactics. One of the most important ways navigators can assist in these efforts is by publishing their original research, thereby helping to demonstrate the value of navigation.
“If you want to publish in a peer-reviewed journal, I speak on behalf of the entire editorial board for JONS when I say that we welcome the opportunity to consider your research for publication,” she said at the 2023 AONN+ Midyear Conference in Orlando.
But at the end of the day, both AONN+ and JONS seek to enrich the literature on navigation topics, and publication in any peer-reviewed oncology nursing journal will help to move the needle in that direction, she added.
“Everyone at this conference knows which navigation tactics work for our patients, but we want to encourage you to prove it,” she said. “Prove it by choosing the navigation tactics that work, building a study around it, and reporting your findings.”
Conducting research in the field of navigation should aim to improve clinical outcomes, improve the patient experience, or demonstrate a positive return on investment of navigation programs.
“We want to encourage you look at navigation tactics, study them and report on them,” she said. “So, why publish? The short answer is, when you publish your study in a peer-reviewed journal, it instantly lends credibility to your research topic.”
Publishing your navigation research allows for the widespread sharing of scientifically relevant information in reputable peer-reviewed forums. It also allows navigators to advance their own professional development while gaining recognition for their institutions, all while advancing the field of navigation.
“But there’s also a personal component,” she noted. “Becoming a published author feels pretty good.”
According to Ms. Siyahian, JONS is more than just a journal: it serves as an extension of the AONN+ community and a vehicle for exchanging best practices established by actual navigators. Published in print and online, JONS promotes evidence-based practices in oncology navigation, disseminates original research findings, and fosters the growing community of oncology navigators.
She pointed out that submitting original research is not the only way to be published in JONS, as the editorial department also welcomes submissions for review articles, commentaries/op-eds, case studies, etc.
Submitting your original research paper for consideration first involves writing an abstract, which should include:
- Background: Why is this topic important?
- Objectives: What do you want to find out? (This should be accomplished in 1-2 sentences.)
- Methods: How did you obtain results?
- Results: What did you discover, reveal, and learn?
- Conclusions: What are the implications of your research?
The abstract is a concise summary of the research conducted, while the actual article details the entirety of the research study. In order to parlay an abstract into a full-fledged original research article to submit to a peer-reviewed journal, authors should make sure they have:
- An introduction with fully referenced background information
- A detailed description of methods employed
- Discussion and interpretation of the results and their impact
- If applicable, figures and/or tables to clarify and display their data, as well as supplemental materials, surveys, etc
“You want to expand the narrative and fully flesh out what you did in your research,” Ms. Siyahian said.
The JONS website includes author guidelines and detailed instructions for submitting a manuscript, and outlines exactly what should be included in each type of submission (ie, original research, commentary, etc). It also serves as a submission portal.
“This process can be intimidating, but the JONS website will be a tremendous resource,” she advised.
Before submission, authors are responsible for ensuring data integrity and accuracy, obtaining written permission to reuse or adapt any table or figure from a previously published article or source, and providing full reference information in a numbered list at the end of the article.
Once submitted to the editorial team, a manuscript undergoes an initial review by a member of the board, then a blinded version of the manuscript (with no identifying information, author names, institutions, etc) is sent to 2 reviewers.
“It’s double-blinded, so the author doesn’t know the reviewers, the reviewers don’t know the authors, and the reviewers don’t know each other,” she explained.
A decision, along with the reviewers’ comments, will then be sent to the corresponding authors. More often than not, the reviewers will ask for changes, updates, clarification, etc, and will also provide feedback and precise direction on how the article can be improved. The manuscript may undergo several rounds of revisions before being accepted.
“I really want to point out that peer-review is a form of mentorship. AONN+ is interested in elevating navigation, but we’re also interested in elevating navigators as researchers and writers,” said Ms. Siyahian. “It’s in all of our best interests to try to get these manuscripts accepted.”
Once accepted for publication, the manuscript is sent to the editorial team for internal proof and edit review, and cleaned up for things like grammar, style, and data accuracy. After final approval by the authors themselves, the manuscript is placed into the JONS journal design, and finally posted on the JONS website, printed in the journal, and shared via email and social media.
Authors are also provided with trackable links to help raise awareness about their published article, and ultimately, to help elevate the cause of navigation.
“What’s in It for Me?” Helping Patients Understand Oncology Clinical Trials

Cancer clinical trials offer patients an opportunity to be treated with the most cutting-edge and promising cancer therapies available, but the majority of patients who are offered these trials still aren’t signing up for them. This might frustrate those in the know, but many patients simply don’t understand what these trials entail. However, navigators can play an important role in educating patients on clinical trials, and making sure they don’t miss out on these potentially life-saving opportunities.
At the 2023 AONN+ Midyear Conference in Orlando, FL, Mary Salazar, DNP, MSN, ANP-BC, RN, and Mike Harris, MBA, provided some simple tools for helping patients better understand clinical trials—perhaps most importantly, that they will not be left with a placebo.
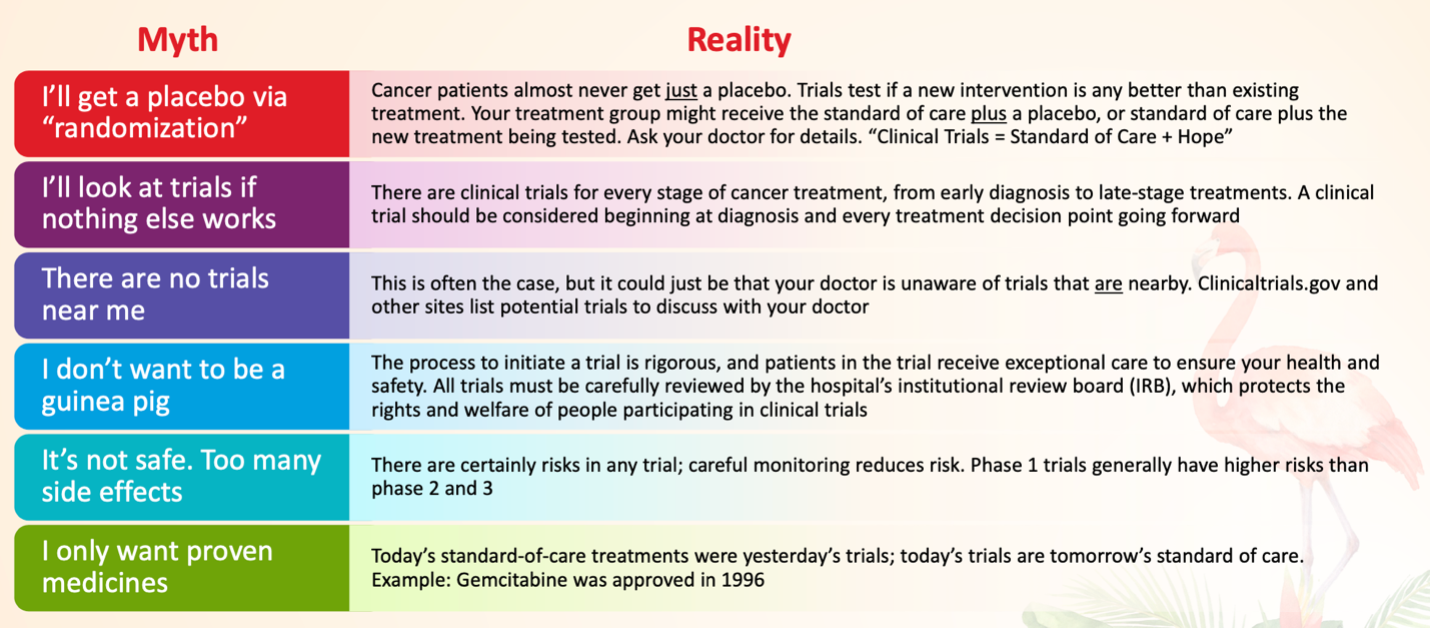
“They might receive the placebo instead of the study drug, but they’ll still be getting the gold standard of care, which they would have gotten had they not been involved in the trial at all,” explained Mr Harris, COO of TriCan Health, LLC, a company that matches patients with available cancer clinical trials. “I can’t tell you how many people I’ve talked to who have no idea that’s how oncology clinical trials work.”
Clinical trials for cancer compare the most effective known treatment for a specific type or stage of cancer with a new approach: either a new drug, a combination of drugs, or a different way of using established therapies.
“The purpose of an oncology clinical trial is to test where we are, in order to get to where we can be,” said Dr Salazar, Director of Oncology Patient Experience and Assistant Professor in the Division of Medical Oncology at UT Health San Antonio, MD Anderson Mays Cancer Center.
Patients should understand that, through these trials, healthcare providers find new ways to improve treatments and quality of life for people with cancer, as they are only comparing the most promising, state-of-the-art treatment to existing standard of care.
The first phase of a clinical trial—phase 1—evaluates safety, determines safe dosage/mode of delivery, and identifies side effects in a small group of patients (<30).
“It’s the first time this treatment is being tested in humans,” she noted. “So there’s a lot of regulation and safety surrounding the whole process.”
Phase 2 trials look at benefit versus risk by testing the effectiveness of the treatment and further evaluating its safety in 100 or fewer patients. Phase 3 trials collect more information, further confirm the effectiveness of the new treatment, and compare it to the current standard of care; these trials can involve thousands of patients, and results may or may not lead to FDA approval of the study drug. Phase 4 (postmarketing) trials are conducted after the drug’s approval, and evaluate long-term side effects.
Ms. Salazar encourages her patients to visit the NCI website (cancer.gov), where they can access informational videos on clinical trials. Additionally, for patients interested in complementary and alternative medicines (CAM), like acupuncture and yoga, a long list of active CAM clinical trials is available at the following link: https://www.cancer.gov/about-cancer/treatment/clinical-trials/cam-procedures. “I use this a lot for my patients who are nervous about clinical trials,” she said.
Clinical trials test tomorrow’s medicine, today. According to Mr Harris, they offer patients a combination of hope and access: hope that there is more than what they perceive is available in terms of cancer treatment, as well as access to these advanced medicines.
Additionally, patients in clinical trials often receive an enhanced level of care, as the entire medical team is invested in each patient enrolled in a trial.
“The physicians involved in these trials are carefully monitoring the patients—they want to make sure there aren’t any adverse side effects, or if there are, that they’re rapidly recognized and treated,” he explained. “So oftentimes patients can actually get better care on a clinical trial than they would receiving standard of care.”
Underrepresented patients who enroll in trials can help to increase access to cutting-edge treatments for entire populations, as they will be helping to test new medicines that may be particularly effective in diverse patient populations. Lastly, patients in clinical trials will ultimately be helping other patients with the same diagnosis.
“The concept of altruism is consistent throughout participants in clinical trials: they want to do what’s right for the whole community, including anyone else who suffers from their particular disease,” he added.
According to Mr Harris, about 75% of patients who are offered cancer clinical trials express interest in learning more, but less than 8% ultimately enroll in those trials. There are a multitude of reasons for this, but many of them involve myths and widespread misinformation about clinical trials, and a common misconception that they’ll be treated as “guinea pigs.”
Helping patients to overcome their concerns over these myths simply involves explaining the realities:
Other concerns around the trial itself might include logistics like too much travel time, but navigators can ask about the treatment protocol for potential trials, as some require less frequent trips to the medical center. Travel resources can be found through the American Cancer Society “Road to Recovery” program, Medicaid Expansion Non-Emergency Transportation Programs (ie, Modivcare, MTM), and services like OncoLink, Ride Health, Envoy, GoGo, and Findhelp.org.
Expense is another commonly cited barrier, but navigators should check the specifics of each trial, as trial sponsors typically pay expenses over and above the standard of care, while insurance or Medicare typically covers standard care costs.
Navigators play a crucial role in helping patients to overcome these barriers, and in encouraging them to consider clinical trials throughout their entire cancer journey. To sum it up, added Mr Harris, “Clinical Trials = Standard of Care + Hope.”
Defining New Roles: What’s an Oncology Clinical Trial Nurse Navigator?

In order to better assist their oncology patients with getting into clinical trials, providers at University of Florida (UF) Health Cancer Center recently decided that they needed a new kind of navigator: an oncology clinical trial nurse navigator.
Like any oncology navigator, this new position is based largely on assessing and addressing barriers to care, according to Jennifer West, BSN, RN, CCRC, ONN-CG, a clinical trials navigator at UF Health Cancer Center, who is also a certified research coordinator, a certified oncology nurse navigator, and Co-Chair of the AONN+ Clinical Trials Committee. But in this particular role, the hurdles addressed are those standing in the way of patient enrollment on oncology clinical trials.
“Getting into a large referral center like ours comes with a lot of barriers and obstacles,” she said at the AONN+ Midyear Conference in Orlando, FL. “So we developed this position, which basically combines both of my skillsets, and we’ve had it now for about a year.”
As the new clinical trial nurse navigator, some of Ms West’s responsibilities include:
- Reviewing current oncology clinic schedules to preidentify potential patients
- Reviewing new patient referrals that are scheduled in the oncology or surgery clinic
- Communicating potential patient eligibility to the research coordinator and research team of each disease-specific group
- Monitoring their cancer center website—“NaviGator”—for inquiries about current ongoing trials
- Assessing barriers that would hinder participation in a clinical trial
According to Ms West, large numbers of patients in the Southeast come to UF Health for second opinions and want to get on clinical trials at her facility, but a multitude of barriers often stand in their way.
“They started this position for me to assess those barriers upfront, and to see if we could help increase enrollment to clinical trials,” she explained.
On their cancer center website, “NaviGator,” patients can see specifically which clinical trials are available according to their cancer type and stage, and can call or send an email directly to Ms West to inquire about any trials they think they might be eligible for. “We get them on the phone within 24 hours, and we talk to them about what we have to offer at our facility,” she said.
During that initial conversation with the patient, Ms West immediately begins to assess any barriers that can be addressed early on, in order to more quickly enroll them into a clinical trial.
She then works with the facility’s research team to obtain any outside records (ie, clinical records, scans, genetic testing, molecular testing, etc), so all records are on hand before the patient even reaches their facility.
An important aspect of her role is communicating with the facility’s research coordinators about potential trial candidates. She prescreens each day’s clinical schedule and lets the research coordinators know which patients look like they would be eligible for an available clinical trial.
“Probably about 80 patients a day come through our oncology clinics,” she said. “By helping them in this way, the research coordinator and the physician are ready for that patient with options when they come back to clinic.”
Once she has identified a patient that is both interested and eligible for a trial—and has addressed any barriers standing in their way—she hands care over to the research coordinator, who facilitates the remaining steps to get the patient onto the clinical trial.
Once the patient is on the trial and the coordinator takes over, the nurse navigator plays an important role in becoming part of the trial process and facilitating communication between other providers, she said.
When it comes to promoting clinical trial enrollment, nurse navigators should first become familiar with any trials enrolling patients in their specific disease type, and at what stage (ie, neoadjuvant, adjuvant, progression after first-line therapy, etc).
“Be a patient advocate by bringing up clinical trials at tumor boards, and by continuously assessing barriers as you work with patients through their treatment process,” she said. Increase their interest (without attempting to persuade them to participate in a trial), and provide them with the basic knowledge to ask questions of their providers, she advised.
Finally, educate patients on clinical trials, even those who are newly diagnosed. According to Ms West, the fundamental basis of a clinical trial and its ability to move forward with discovering new treatments is patient participation. But first there must come patient education, at which point navigators are invaluable.
“A clinical trial doesn’t have to be the last hope for these patients. It can be the first step in their care,” she said. “This is their cancer journey; let’s do everything we can to get them into survivorship.”